10 year anniversary event of the public-private partnership “london declaration”
eisai co., ltd. (headquarters: tokyo, ceo: haruo naito, “eisai”) announced today that its ceo haruo naito participated in the online event entitled “100% committed to end ntds” celebrating the 10th anniversary of the london declaration, an international public-private partnership to eliminate neglected tropical diseases (ntds), on january 27, 2022. ceo naito represented pharmaceutical companies and highlighted the achievements of ntds elimination activities by the industry and successes achieved by multi-sectoral partnerships. he also expressed eisai’s continued support for the elimination of ntds towards the achievement of ntd road map 2021-2030 launched by the world health organization (who) last year. the 10th anniversary event aimed to recognize a decade of progress since the london declaration, to confirm the strong commitment of stakeholders to continue the efforts for ntds, and to strengthen endorsements from stakeholders to the kigali declaration on ntds, the successor of the london declaration which will be unveiled at the commonwealth heads of government meeting (chogm) scheduled for june 2022 in kigali, the capital of the republic of rwanda.
tremendous achievements have been made through public-private partnership since the launch of the london declaration to 2020. the pharma industry has contributed to the elimination of ntds via supply of medicines which resulted in donation of 13 billion high-quality treatments. forty-three countries have eliminated at least one ntd and 600 million people no longer require interventions against ntds. despite such progress, more than 1.7 billion people remain threatened by ntds.
in the event, ceo haruo naito said “our initiatives toward ntds elimination are rooted in the pharmaceutical company’s fundamental mission to deliver medicines to those who need them to treat illness and save lives. while r&d for ntds treatments have become more active after the london declaration, establishment of agile and flexible regulatory approval system as well as expansion of funding which leverages public-private partnership will be required to accelerate further innovations. utilization of digital technologies such as remote medicine will help ensure delivery of and access to healthcare service under the vulnerable social infrastructure.”
under the london declaration, eisai has been manufacturing and supplying diethylcarbamazine (dec) tablets, one of the lymphatic filariasis (lf) treatments, free of charge to the who for the elimination of lf. to date, 2.05 billion dec tablets manufactured at eisai’s vizag plant in india have been supplied to 29 countries (as of january 2022). although lf has been eliminated in 17 countries and the number of infected people has declined by 74% since 2000, 860 million people worldwide are still exposed to the risk of infection. eisai is committed to providing dec tablets for free to endemic countries that need dec until lf is eliminated in these countries. in addition to the supply of dec tablets, eisai is working on various initiatives such as support for the mass drug administrations (mda), disease awareness and improvements in sanitation.
furthermore, eisai is proactively engaged in development of new treatments for ntds through partnerships with global research organizations. utilizing the funding from the global health innovative technology fund (ghit fund) and others, eisai is conducting joint development of new treatments such as a new treatment for lf in collaboration with the liverpool school of tropical medicine and the university of liverpool as well as treatments for mycetoma and leishmaniasis in collaboration with the drugs for neglected diseases initiative (dndi).
eisai commits to the kigali declaration and strengthens collaborations with global partners to tackle ntds towards the achievement of ntd road map 2021-2030.
based on human health care (hhc) philosophy, eisai seeks to contribute to the health and welfare of people in developing and emerging countries. once they recover their health, they can resume productive activities, which will in turn contribute to economic development and expansion of the middle-income class. eisai considers this to be a long-term investment in creating the markets for the future. eisai is actively engaged in leveraging partnerships with governments, international organizations, academia, and non-profit private sector organizations to accelerate the development of new treatments for infectious diseases including ntds and facilitate initiatives to improve access to medicine such as support for mdas and disease awareness activities. through these initiatives, eisai seeks to further contribute to patients and their families worldwide and increase the benefits that health care provides.
media inquiries:
public relations department,
eisai co., ltd.
81-(0)3-3817-5120
[notes to editors]
1. about neglected tropic diseases (ntds)
neglected tropic diseases (ntds) include 20 diseases that the who identifies as tropical diseases which human race must overcome. more than 1.7 billion people living in the poorest and most marginalized communities worldwide are exposed to the risk of ntds infection. the spread of ntds is mainly caused by poor hygienic conditions associated with poverty. infections from these diseases may result in serious physical impairment and this often results in normal economic and social activities becoming highly challenging to the individual. in the worst cases, ntds may also result in death. the prevalence of ntds is a stumbling block to economic growth for developing and emerging countries and represents a serious issue for these regions.
the following 20 ntds have been designated by who for control or elimination: dengue and chikungunya, rabies, trachoma, buruli ulcer, yaws (endemic treponematoses], leprosy (hansen’s disease], chagas disease, human african trypanosomiasis (sleeping sickness], leishmaniasis, taeniasis / cysticercosis, dracunculiasis (guinea-worm disease), echinococcosis, food-borne trematodiases, lymphatic filariasis, onchocerciasis (river blindness], schistosomiasis, soil-transmitted helminthiases, mycetoma, scabies and other ectoparasites, and snakebite envenoming.
2. about london declaration on neglected tropical diseases
on january 30, 2012, the ceos of 13 pharmaceutical companies*, the bill & melinda gates foundation, who, the u.s. agency for international development (usaid), the u.k. department for international development (dfid), the world bank, and officials from ntd-endemic countries gathered in london to pledge their support for a coordinated effort to combat 10 ntds**. the london declaration represents the largest international public-private partnership in the field of global health to date, and unlike past approaches undertaken by an individual organization or for a single disease, the group has committed itself to working collaboratively in an effort to comprehensively tackle issues pertaining to drug supply, distribution, development, and implementation programs as it seeks to more effectively combat ntds.
commemorating the london declaration, january 30 has been designated as the world ntd day since 2020 and campaigns are held worldwide to light up the iconic landmarks and monuments in orange and purple, the symbol colors of ntds. eisai is sponsoring the light up of tokyo tower to raise disease awareness and disseminate the importance of eliminating ntds. (please click for the details of the world ntd day.)
* abbvie, astrazeneca, bayer, bristol-myers squibb, eisai, glaxonsmithkline, gilead, johnson & johnson, merck (merck kgaa: germany), merck sharp & dhome, novartis, pfizer, sanofi
** guinea worm disease, lymphatic filariasis, blinding trachoma, sleeping sickness (human african trypanosomiasis], leprosy, soil-transmitted helminthes, schistosomiasis, river blindness, chagas disease, and visceral leishmaniasis
3. about kigali declaration on neglected tropical diseases
as the successor of the london declaration on ntds launched in 2012, the kigali declaration represents a collective commitment from stakeholders to fight against ntds. with the endorsements from stakeholders being initiated at the online event entitled “100% committed to end ntds”, a campaign to commemorate the 10th year anniversary of the london declaration held on january 27, 2022, the kigali declaration will be unveiled at the commonwealth heads of government meeting (chogm) scheduled for june 2022 in kigali, the capital of the republic of rwanda. to achieve the who’s ntd roadmap 2021-2030, the kigali declaration aims to tackle ntds comprehensively and sustainably by sustaining a multisectoral and multidisciplinary approach through public-private partnership, strengthening country ownership including establishment of local health system and domestic financing, accelerating research and development of treatments and diagnostics for ntds and ensuring equitable access to these ntds related products and services.
4. about lymphatic filariasis
lymphatic filariasis (lf) is an ntd transmitted to humans via carrier mosquitoes. lf causes lymphatic dysfunction and can lead to the swelling of body parts such as legs, and cause severe pain, permanent disability and social stigma associated with disfiguring visible manifestations. as a result, patients suffer mental, social and financial losses. it is estimated that 860 million people worldwide, mainly those in developing countries, are exposed to the risk of lf. elimination of lf is possible by stopping the spread of the infection through mdas of three types of lf treatments including dec tablets.
5. about eisai’s commitment to improving global access to medicines including lf elimination program
in line with its hhc philosophy, eisai is committed to improving global access to medicines over the medium-to-long term through partnership strategies that involve working with governments, international organizations, private entities and non-profit organizations.
in november 2010, eisai agreed to supply a total of 2.2 billion dec tablets to the who free of charge by 2020, as there was a global shortage of high-quality dec tablets for use in mdas. in 2012, eisai became the only japanese company to participate in the london declaration, a coordinated effort to eliminate 10 ntds and the largest public-private partnership of its kind in the field of global health. at the london declaration’s fifth anniversary event held in april 2017, eisai announced its plan to supply dec tablets continuously beyond 2020, until lf is eliminated in all endemic countries where dec tablets are needed.
eisai has supplied 2.05 billion tablets to 29 countries through the who’s elimination program (as of january 2022). furthermore, in order to support the smooth implementation of the who’s mda programs, eisai is engaging in initiatives to raise public awareness of lf in endemic countries. staff members of eisai group cooperate with the relevant representatives in endemic countries to eliminate lf as early as possible.
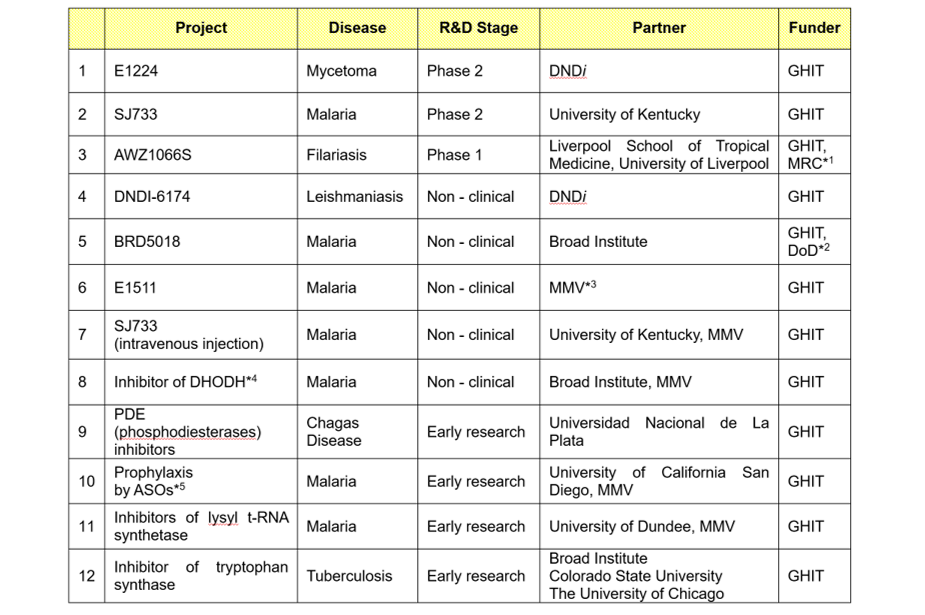
in addition to the above-mentioned initiatives, eisai is moving ahead with new drug development projects targeting malaria and ntds such as mycetoma and lf, based on partnerships with international non-profit organizations such as the drugs for neglected diseases initiative (dndi) and medicines for malaria venture (mmv), as well as research organizations such as liverpool school of tropical medicine, university of kentucky, and the broad institute (please refer to the table below).
furthermore, eisai co-established the global health innovative technology fund (ghit fund), japan’s first public–private partnership to advance development of new health technologies for the developing world, is a member of the world intellectual property organization (wipo) re:search consortium, an international joint enterprise for the development of treatments for ntds, malaria and tuberculosis led by wipo, is a signatory to the tuberculosis drug accelerator (tbda) partnership, and is participating in the access accelerated initiative to promote prevention and treatment of non-communicable diseases.
*1 mrc: medical research council (uk); *2 dod: department of defense (us); *3 mmv: medicines for malaria venture (swiss); *4 dhodh: dihydroorotate dehydrogenase; *5 aso: anti-sense oligo-nucleotide
for further information on eisai’s access to medicines initiatives, please visit the access to medicines page on the eisai global website: