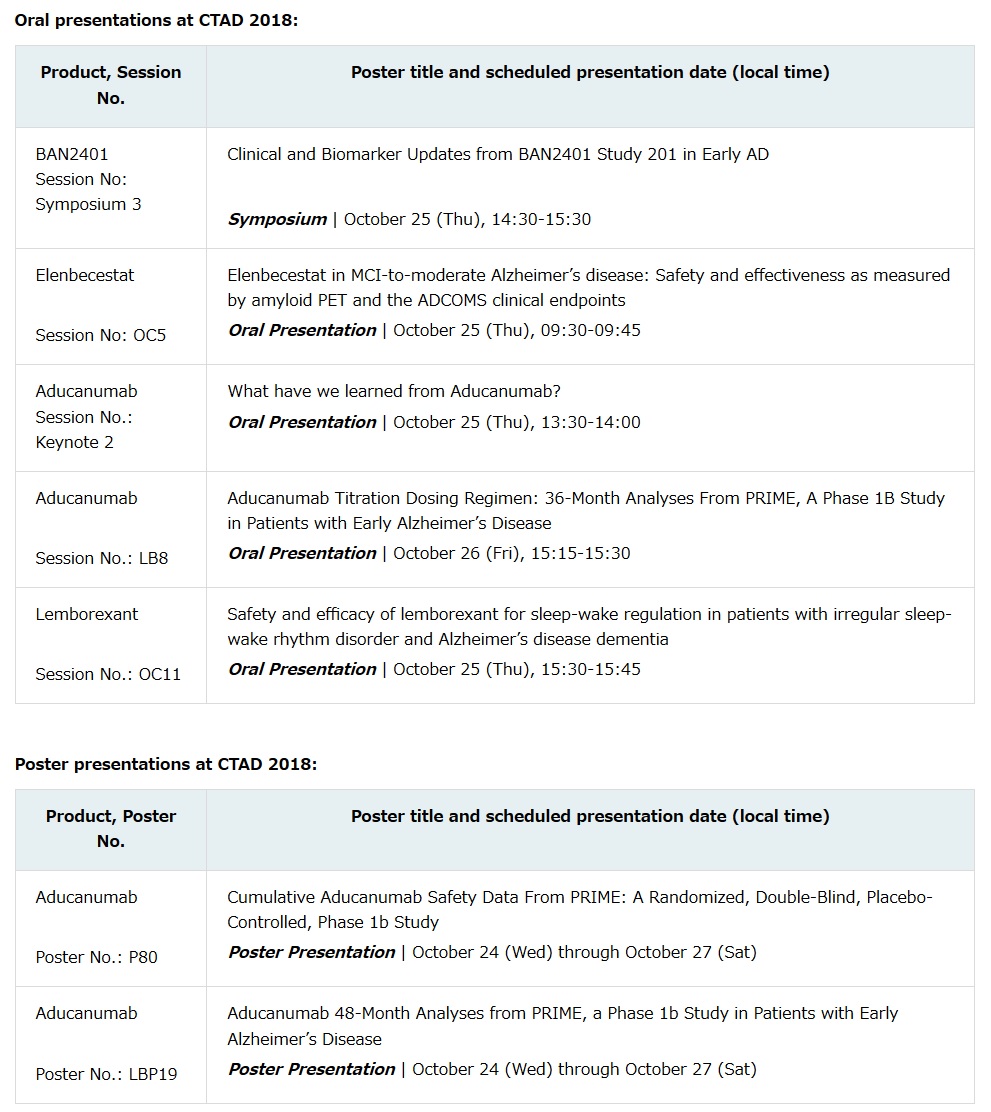
eisai co., ltd. (headquarters: tokyo, ceo: haruo naito, “eisai”) and biogen inc. (nasdaq: biib) (headquarters: cambridge, massachusetts, united states, ceo: michel vounatsos, “biogen”) announced that eisai presented the latest data from the phase ii clinical study (study 201) of ban2401, an anti-amyloid beta protofibril antibody, in 856 patients with early alzheimer’s disease, at a symposium session titled “clinical and biomarker updates from ban2401 study 201 in early alzheimer’s disease” held on october 25 at the 11th clinical trials on alzheimers disease (ctad) conference in barcelona, spain.
study 201 is a placebo-controlled, double-blind, parallel-group, randomized study in 856 patients with mild cognitive impairment (mci) due to alzheimer’s disease (ad) or mild alzheimer’s dementia (collectively known as early alzheimer’s disease) with confirmed amyloid pathology in the brain. patients were randomized to five dose regimens, 2.5 mg/kg bi-weekly, 5 mg/kg monthly, 5 mg/kg bi-weekly, 10 mg/kg monthly and 10 mg/kg bi-weekly, or placebo. this study used a bayesian adaptive randomization design to automatically allocate newly enrolled patients into the study to treatment arms showing higher probability of efficacy based on the results of interim analyses. the 10 mg/kg monthly and 10 mg/kg bi-weekly doses were determined to have greater efficacy, and as a result, the proportion of patients allocated to those treatment arms was greater.
conventional statistical methods on predefined clinical outcomes at the 18 month final efficacy time point included alzheimer’s disease composite score (adcoms), alzheimer’s disease assessment scale-cognitive subscale (adas-cog), and clinical dementia rating sum of boxes (cdr-sb).
the most current data presented at ctad 2018 highlight topline results, originally presented by eisai in july at the alzheimer’s association international conference (aaic) 2018, as well as new data from pre-specified subgroup analyses and cerebrospinal fluid (csf) biomarkers. the full ctad presentation is available on the investor relations section of the eisai website.
from conventional statistical analysis of the topline results, the highest treatment dose demonstrated a statistically significant reduction in brain amyloid measured by positron emission tomography (pet) at 18 months (p<0.0001). this dose also showed a statistically significant slowing of clinical decline on adcoms of 30 percent compared to placebo at 18 months (p=0.034). a group-level correlation between clearance of brain amyloid and slowing of clinical decline on adcoms was confirmed (pearson’s correlation coefficient of 0.838). a linear regression model testing the slope of change from baseline on the rate of disease progression using adcoms showed a significant difference over 18 months (p<0.001) for the highest treatment dose versus placebo, suggesting a potential disease-modifying effect.
a request from a health authority in july 2014 required an amendment to be implemented restricting enrollment of apoe4 carriers in the highest treatment dose arm (10 mg/kg bi-weekly), resulting in an imbalance of apoe4 carriers in that arm versus placebo. to assess the influence of apoe4 status on the observed effect in the highest treatment dose, the rates of clinical decline for apoe4 carriers and non-carriers in the placebo group were analyzed and shown not to be statistically significantly different from each other on adcoms, adas-cog and cdr-sb. analysis on clinical outcome measures was also conducted in pre-specified subgroups of apoe4 status. at the highest treatment dose, apoe4 carriers treated with ban2401 saw 63% less decline in disease progression, while non-carriers saw 7% less decline, as measured by adcoms versus placebo at 18 months. these results suggest that the treatment effect for the 10 mg/kg bi-weekly dose is related to treatment with ban2401 and not due to an imbalance in subject allocation by apoe4 status. in addition, the pooled 10 mg/kg bi-weekly and 10 mg/kg monthly doses result in less decline on adcoms versus placebo at 18 months (overall; 21%, apoe4 carriers; 25%, apoe4 non-carriers; 6%). more detailed results were presented at ctad.
analyses of clinical outcome measures on pre-specified subgroups, by clinical stage and by use of concomitant alzheimer’s disease medications, were also conducted. treatment with the highest treatment dose also resulted in less decline in disease progression on adcoms at 18 months versus placebo across subgroups of clinical stage (mci due to ad subgroup; 33% and mild ad subgroup; 35%) and use of concomitant alzheimer’s disease medications (with concomitant ad meds; 23% and without concomitant ad meds; 41%). the study was not powered to show statistical significance in subgroups.
exploratory data on csf biomarkers of neurodegeneration that are elevated in ad were also presented by eisai. to increase the sample size of the csf subgroup, analyses were conducted on samples from the combined 10 mg/kg bi-weekly and 10 mg/kg monthly cohorts. from the results, markers of synaptic damage (neurogranin), tau pathology (phosphorylated-tau, p-tau), and axonal degeneration (neurofilament light chain, nfl) showed trends that are suggestive of impact on underlying disease pathophysiology.
ban2401 demonstrated an acceptable tolerability profile through 18 months of study drug administration. the most common treatment emergent adverse events were infusion-related reactions and amyloid-related imaging abnormalities (aria), both of which were dose-dependent. incidence of aria-e (edema) was greater in apoe4 carriers. ten percent of aria-e cases (5 of 48 patients) were symptomatic and included headache, visual disturbances, and confusion. sixty percent of aria-e occurred within the first three months of treatment and approximately 89 percent of cases were mild to moderate in severity.
eisai and biogen are currently discussing the next steps for ban2401 with regulatory authorities. an open-label extension for patients previously enrolled in study 201 is being planned, with enrollment expected to begin this year.
this release discusses investigational uses of an agent in development and is not intended to convey conclusions about efficacy or safety. there is no guarantee that any investigational uses of such product will successfully complete clinical development or gain health authority approval.
biogen safe harbor statement
this press release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the private securities litigation reform act of 1995 about results from the phase 2 study of ban2401; the potential clinical effects of ban2401; risks and uncertainties associated with drug development and commercialization; the potential benefits, safety and efficacy of ban2401 and therapies for other neurological diseases; the timing and status of current and future regulatory filings; the anticipated benefits and potential of biogen’s collaboration arrangements with eisai; and the potential of biogen’s commercial business and pipeline programs, including ban2401, elenbecestat and aducanumab. these forward-looking statements may be accompanied by words such as “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “plan,” “potential,” “possible,” “will,” and other words and terms of similar meaning. drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. results in early stage clinical trials may not be indicative of full results or results from later stage or larger scale clinical trials and do not ensure regulatory approval. you should not place undue reliance on these statements or scientific data presented.
these statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including without limitation, unexpected concerns that may arise from additional data, analysis, or results obtained during clinical trials; regulatory authorities may require additional information or further studies, or may fail or refuse to approve or may delay approval of biogen’s drug candidates, including ban2401, elenbecestat, and/or aducanumab; the occurrence of adverse safety events; risks of unexpected costs or delays; the risks of other unexpected hurdles; uncertainty of success in the development and potential commercialization of ban2401, elenbecestat, and/or aducanumab, which may be impacted by, among other things, unexpected concerns that may arise from additional data or analysis, the occurrence of adverse safety events, failure to obtain regulatory approvals in certain jurisdictions, failure to protect and enforce biogen’s data, intellectual property and other proprietary rights, and uncertainties relating to intellectual property claims and challenges; uncertainty as to whether the anticipated benefits and potential of biogen’s collaboration arrangement with eisai can be achieved; product liability claims; and third party collaboration risks. the foregoing sets forth many, but not all, of the factors that could cause actual results to differ from biogen’s expectations in any forward-looking statement. investors should consider this cautionary statement, as well as the risk factors identified in biogen’s most recent annual or quarterly report and in other reports biogen has filed with the securities and exchange commission. these statements are based on biogen’s current beliefs and expectations and speak only as of the date of this press release. biogen does not undertake any obligation to publicly update any forward-looking statements, whether as a result of new information, future developments, or otherwise.
media inquiries
1. about ban2401
ban2401 is a humanized monoclonal antibody for alzheimer’s disease that is the result of a strategic research alliance between eisai and bioarctic. ban2401 selectively binds to neutralize and eliminate soluble, toxic aβ aggregates that are thought to contribute to the neurodegenerative process in alzheimer’s disease. as such, ban2401 may have the potential to have an effect on disease pathology and to slow down the progression of the disease. eisai obtained the global rights to study, develop, manufacture and market ban2401 for the treatment of alzheimer’s disease pursuant to an agreement concluded with bioarctic in december 2007. in march 2014 eisai and biogen entered into a joint development and commercialization agreement for ban2401 and the parties amended that agreement in october 2017.
2. about study 201
study 201 is a placebo-controlled, double-blind, parallel-group, randomized phase ii clinical study in 856 patients with mild cognitive impairment (mci) due to alzheimer’s disease or mild alzheimer’s dementia (collectively known as early alzheimer’s disease) with confirmed amyloid pathology in the brain. this study used bayesian adaptive randomization design to automatically allocate newly enrolled patients into the study to treatment arms showing higher probability of efficacy based on the results of interim analyses. the study design included five dose regimens and placebo, and considered the efficacy of ban2401 as well as dose responsiveness through 16 interim analyses that assessed potential for early success, an analysis based on adcoms at 12 months (primary endpoint), and a comprehensive final analysis at 18 months (secondary endpoints). patients who received treatment with ban2401 were randomized to five dose regimens, 2.5 mg/kg biweekly (52 patients), 5 mg/kg monthly (51 patients), 5 mg/kg biweekly (92 patients), 10 mg/kg monthly (253 patients), or 10 mg/kg biweekly (161 patients). biomarker endpoints included changes in aβ accumulated in the brain as measured by amyloid pet (positron emission tomography) as well as in cerebrospinal fluid (csf), while adcoms (alzheimer’s disease composite score), clinical dementia rating sum of boxes (cdr-sb) and alzheimer’s disease assessment scale-cognitive subscale (adas-cog) were measured as efficacy endpoints (clinical).
3. about adcoms
developed by eisai, adcoms (ad composite score) combines items from the adas-cog (alzheimer’s disease assessment scale-cognitive subscale), cdr-sb (clinical dementia rating sum of boxes) and the mmse (mini-mental state examination) scales to enable a sensitive detection of changes in clinical functions of early ad symptoms and changes in memory. this study 201 utilizes adcoms as its key endpoint for assessing clinical symptoms.
4. about amyloid pet imaging
amyloid pet (positron emission tomography) imaging is a diagnostic method that enables the visualization of amyloid plaque present in the brain as well as the quantitative evaluation of amyloid plaque distribution and accumulation in the brain via administration of a minute amount of pet tracer, which specifically binds to amyloid plaque. amyloid pet imaging enables the assessment of pathology change and assistance of diagnosis of patients with alzheimer’s disease, including mci due to ad, and could predict clinical response.
5. about correlation coefficient
the correlation coefficient indicates the strength of the relationship between two variables from two quantitative data distributions. the correlation coefficient ranges in value from -1 to 1, and as it approaches the absolute value of 1, it indicates a total positive linear correlation. in general, if a correlation coefficient is 0.6 or greater, it suggests there is a relationship between the variables.
6. about the joint development agreement between eisai and biogen for alzheimer’s disease
eisai and biogen are widely collaborating on the joint development and commercialization of alzheimer’s disease treatments. eisai serves as the lead in the co-development of elenbecestat, a bace inhibitor, and ban2401, an anti-amyloid beta (aβ) protofibril antibody, while biogen serves as the lead for co-development of aducanumab, biogen’s investigational anti-amyloid beta (aβ) antibody for patients with alzheimer’s disease, and the companies plan to pursue marketing authorizations for the three compounds worldwide. if approved, the companies will also co-promote the products in major markets, such as the united states, the european union and japan.
as to ban2401 and elenbecestat, both companies will equally split overall costs, including research and development expenses. eisai will book all sales for elenbecestat and ban2401 following marketing approval and launch, and profits will be equally shared between the companies.
7. about eisai co., ltd.
eisai co., ltd. is a leading global research and development-based pharmaceutical company headquartered in japan. we define our corporate mission as “giving first thought to patients and their families and to increasing the benefits health care provides,” which we call our human health care (hhc) philosophy. with approximately 10,000 employees working across our global network of r&d facilities, manufacturing sites and marketing subsidiaries, we strive to realize our hhc philosophy by delivering innovative products to address unmet medical needs, with a particular focus in our strategic areas of neurology and oncology.
leveraging the experience gained from the development and marketing of aricept®, a treatment for alzheimer’s disease and dementia with lewy bodies, eisai has been working to establish a social environment that involves patients in each community in cooperation with various stakeholders including the government, healthcare professionals and care workers, and is estimated to have held over ten thousand dementia awareness events worldwide. as a pioneer in the field of dementia treatment, eisai is striving to not only develop next generation treatments but also to develop diagnosis methods and provide solutions.
for more information about eisai co., ltd., please visit .
8. about biogen
at biogen, our mission is clear: we are pioneers in neuroscience. biogen discovers, develops and delivers worldwide innovative therapies for people living with serious neurological and neurodegenerative diseases. one of the world’s first global biotechnology companies, biogen was founded in 1978 by charles weissmann, heinz schaller, kenneth murray and nobel prize winners walter gilbert and phillip sharp, and today has the leading portfolio of medicines to treat multiple sclerosis, has introduced the first and only approved treatment for spinal muscular atrophy and is focused on advancing neuroscience research programs in alzheimer’s disease and dementia, multiple sclerosis and neuroimmunology, movement disorders, neuromuscular disorders, pain, ophthalmology, neuropsychiatry and acute neurology. biogen also manufactures and commercializes biosimilars of advanced biologics.
we routinely post information that may be important to investors on our website at . to learn more, please visit and follow us on social media – , , , .
9. about bioarctic ab
bioarctic ab (publ) is a swedish research-based biopharma company focusing on disease modifying treatments and reliable biomarkers and diagnostics for neurodegenerative diseases, such as alzheimer’s disease and parkinson’s disease. the company also develops a potential treatment for complete spinal cord injury. bioarctic focuses on innovative treatments in areas with high unmet medical needs. the company was founded in 2003 based on innovative research from uppsala university, sweden. collaborations with universities are of great importance to the company together with our strategically important global partners in the alzheimer (eisai) and parkinson (abbvie) projects. the project portfolio is a combination of fully funded projects run in partnership with global pharmaceutical companies and innovative in-house projects with significant market- and out-licensing potential. bioarctic’s b-share is listed on nasdaq stockholm mid cap (sto:bioa b). .
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

.jpg)