eisai to present latest data on perampanel at 72nd american epilepsy society annual meeting – eisai china lnc.-爱游戏app官网入口
eisai co., ltd. (headquarters: tokyo, ceo: haruo naito, “eisai”) announced today that the latest data on its antiepileptic drug (aed) perampanel (product name: fycompa®) will be presented at the 72nd american epilepsy society annual meeting (aes 2018) to be held from november 30 to december 4, 2018 in new orleans in the united states.
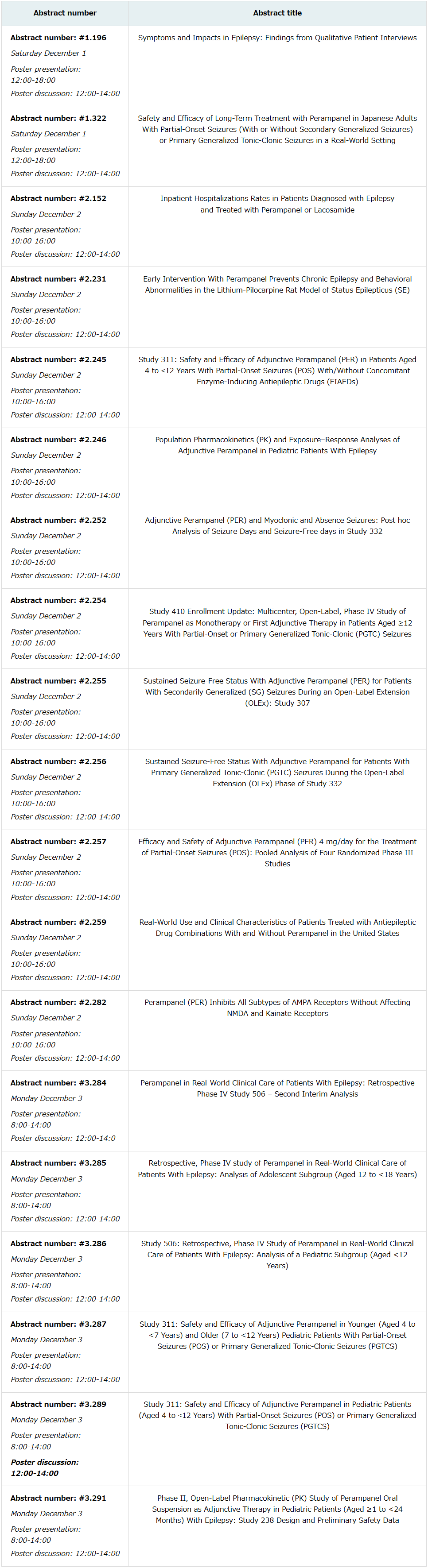
nineteen poster presentations will be given by eisai at aes 2018, including reports on the interim analysis results of a phase iii clinical study (study 311) in pediatric patients aged 4 to less than 12 years as well as a presentation on sustained seizure-free status in the open-label extension phase of a phase iii clinical study (study 307) in patients 12 years of age and older. including investigator initiated studies, more than 50 scientific posters on perampanel will be presented at aes 2018.
perampanel is a first-in-class aed discovered at eisai’s tsukuba research laboratories. it is available in tablet form to be taken once daily, and a new oral suspension formulation has been approved and is being marketed in the united states. a highly selective, noncompetitive ampa receptor antagonist that reduces neuronal hyperexcitation by targeting glutamate activity at ampa receptors on postsynaptic membranes, it is approved in countries around the world as an adjunctive therapy for the treatment of partial-onset seizures with or without secondarily generalized seizures, and primary generalized tonic-clonic seizures in patients with epilepsy 12 years of age and older. furthermore, perampanel is also indicated for monotherapy and adjunctive use in the treatment of partial-onset seizures with or without secondarily generalized seizures in patients with epilepsy 4 years of age and older in the united states.
eisai considers neurology including epilepsy, a therapeutic area of focus, and strives to deliver perampanel throughout the world in pursuit of our mission to provide “seizure freedom” to a greater number of patients living with epilepsy. eisai seeks to address the diverse needs of, as well as increasing the benefits provided to, patients with epilepsy and their families.
major poster presentations for perampanel:
media inquiries:
public relations department,
eisai co., ltd.
81-(0)3-3817-5120