eisai delivers new data and highlights continued progress of oncology portfolio and pipeline at asco 2023 – eisai china lnc.-爱游戏app官网入口
eisai co., ltd. (headquarters: tokyo, ceo: haruo naito, “eisai”) announced today the presentation of research across various types of cancer from its oncology portfolio and pipeline during the 2023 american society of clinical oncology (asco) annual meeting (#asco23), which is taking place virtually and in-person in chicago, illinois from june 2 to 6.
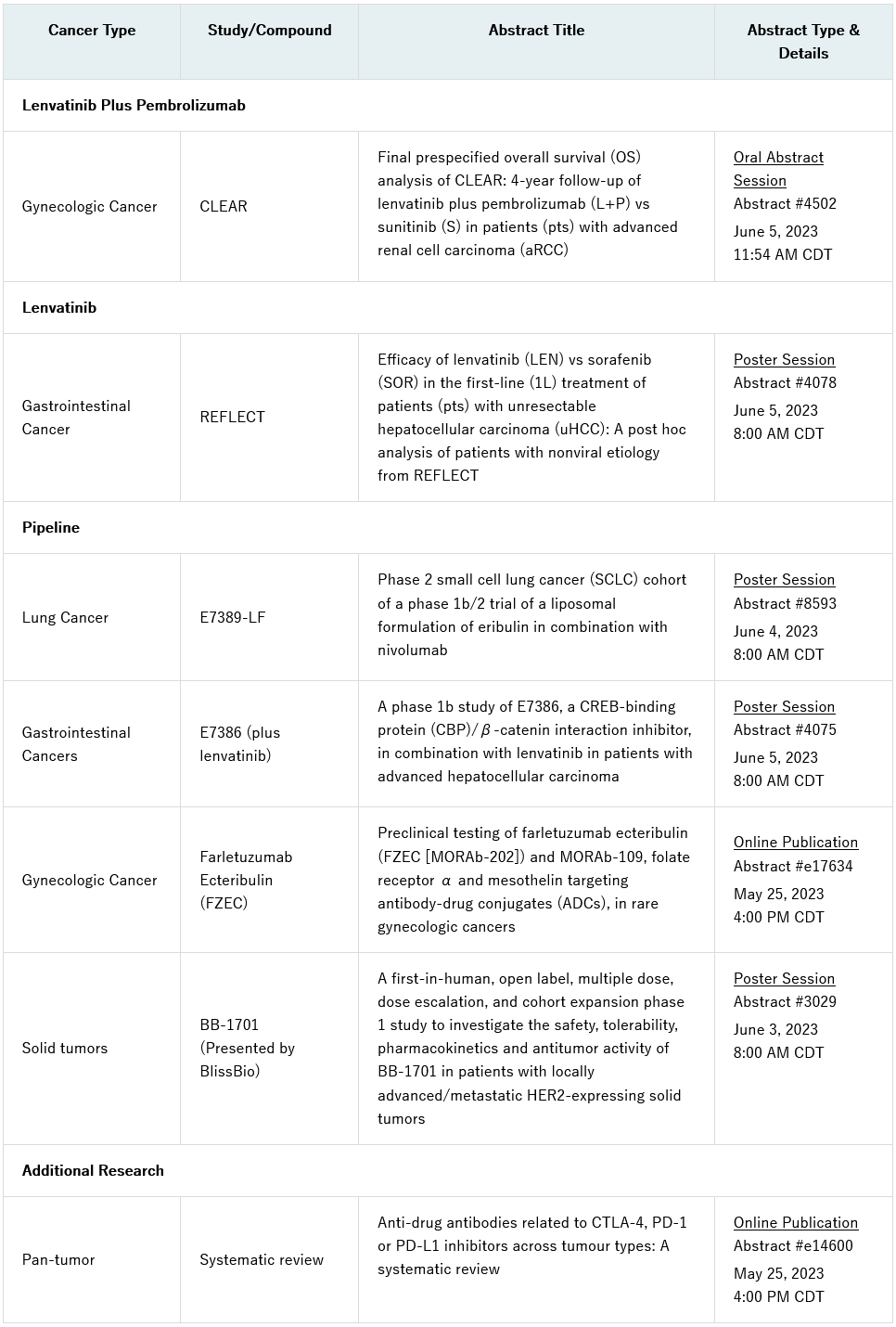
notable research includes an oral presentation of results from the final pre-specified overall survival analysis of the pivotal phase 3 clear (study 307)/keynote-581 trial, which evaluated lenvatinib (lenvima®) plus pembrolizumab (keytruda®) versus sunitinib for the first-line treatment of patients with advanced renal cell carcinoma (abstract #4502). a post hoc analysis from the reflect trial evaluating lenvatinib monotherapy versus sorafenib in the first-line treatment of patients with unresectable hepatocellular carcinoma (hcc) will also be shared in a poster presentation (abstract #4078).
“the outlook for advanced renal cell carcinoma has evolved in recent years, and the final analysis from the pivotal clear trial to be presented at asco represents another step forward for patients and an opportunity to provide their physicians with long-term data,” said dr. takashi owa, chief scientific officer, senior vice president, eisai co., ltd. “new data for lenvatinib and from our oncology pipeline showcase eisai’s continued commitment to driving innovation and exploring novel therapeutic modalities in our ambition to live out our human health care concept, our corporate mission to meet the needs of more people who face a cancer diagnosis.”
additional data from eisai’s pipeline include a poster presentation of findings from a phase 1b study of e7386, a creb-binding protein (cbp) / β-catenin interaction inhibitor, in combination with lenvatinib in patients with advanced hcc (abstract #4075), and the small cell lung cancer cohort of a phase 1b/2 trial evaluating e7389-lf, a new liposomal formulation of eribulin, in combination with nivolumab (abstract #8593). insights from preclinical testing of farletuzumab ecteribulin (fzec), formerly known as morab-202, and morab-109, antibody drug conjugates (adc), in rare gynecologic cancers will also be published online (abstract # e17634).
furthermore, bliss biopharmaceutical co., ltd. (blissbio) will present a poster at the conference with results from the first-in-human study of bb-1701, a her2-targeting adc (abstract #3029). eisai entered into a joint development agreement with blissbio for bb-1701 with option rights for a strategic collaboration in april 2023. a phase 1/2 clinical study of bb-1701 in the u.s. and china for her2-expressing solid tumors is currently underway.
this release discusses investigational compounds and investigational uses for fda-approved products. it is not intended to convey conclusions about efficacy and safety. there is no guarantee that any investigational compounds or investigational uses of fda-approved products will successfully complete clinical development or gain fda approval.
the full list of presentations is included below. these abstracts will be made available on thursday, may 25, 2023 at 4:00 pm central daylight time (cdt).
media inquiries:
public relations department,
eisai co., ltd.
81-(0)3-3817-5120